The role of ApoE Knockout Mice in cardiovascular and neurodegenerative disease research
The role of ApoE Knockout Mice in cardiovascular and neurodegenerative disease research
The Apolipoprotein E (ApoE) gene plays a crucial role in how our bodies handle cholesterol and lipids. In humans, there are three versions of the ApoE gene: ApoE2, ApoE3, and ApoE4. While ApoE3 is considered the “normal” variant, the ApoE4 version is linked to higher risks of Alzheimer’s disease (AD) and cardiovascular problems. Understanding how ApoE works and what happens when it doesn’t function properly is key to unlocking treatments for these serious health conditions.
To help scientists study the effects of ApoE, researchers often use ApoE knockout mice that have been genetically engineered to lack the ApoE gene entirely. These mice serve as incredibly valuable tools for learning about the biological processes that contribute to diseases like Alzheimer’s and heart disease. By observing how these mice develop and how they respond to treatments, researchers can gain important insights into the mechanisms behind these diseases, ultimately leading to better therapies for humans.
What Are ApoE Knockout Mice Used for in Research?
ApoE knockout mice are used in a wide range of research to explore how the ApoE gene impacts the body. By removing the gene entirely, these mice develop symptoms that are similar to the diseases humans experience, particularly in the brain and the cardiovascular system. These mice have helped researchers make major strides in understanding lipid metabolism, inflammation, and how the body’s blood vessels function.
Because these mice mimic the effects of ApoE deficiency, they are incredibly useful in testing new drugs and treatments aimed at diseases like Alzheimer’s and atherosclerosis. Researchers can test potential therapies in these mice before they move on to human trials, ensuring that the treatments are safe and effective.
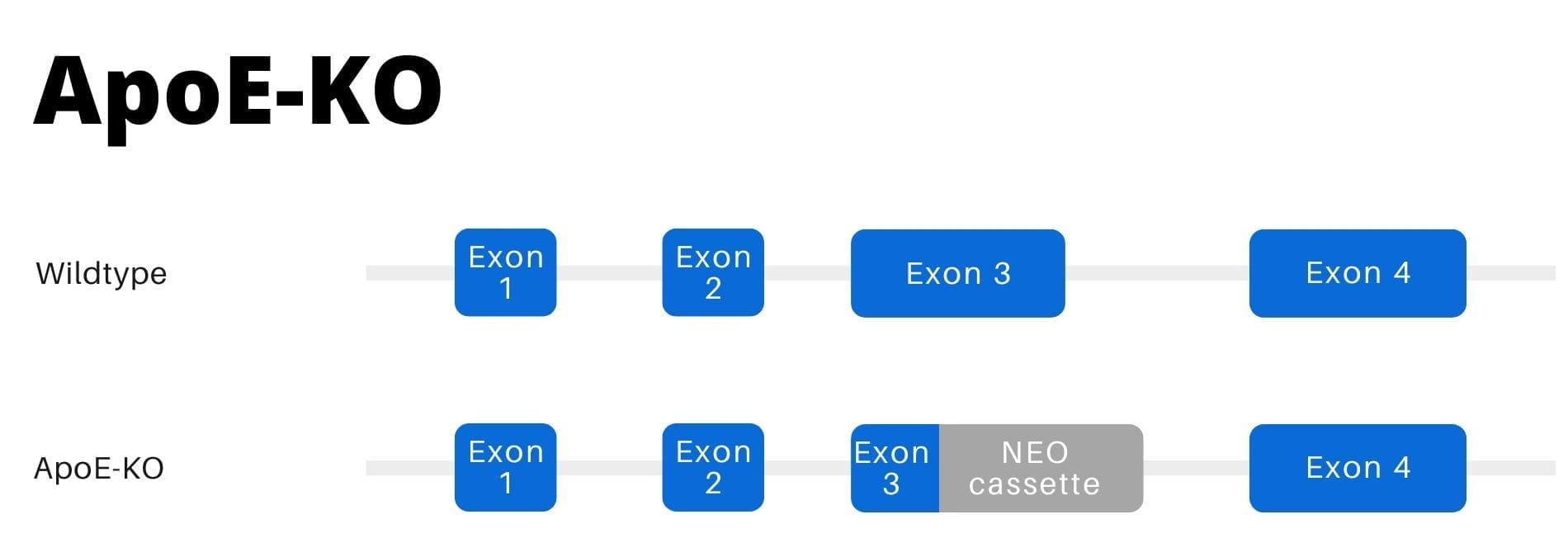
How Do ApoE Knockout Mice Contribute to Alzheimer’s Disease Studies?
Alzheimer’s disease (AD) is a devastating condition that causes memory loss and cognitive decline. One of the key features of AD is the buildup of amyloid-beta plaques in the brain. These plaques contribute to brain damage, and researchers believe they play a major role in the disease. The ApoE4 version of the gene is particularly bad at clearing amyloid-beta, and as a result, people who carry the ApoE4 allele are more likely to develop AD.
ApoE knockout mice have been vital for understanding how the absence of ApoE impacts amyloid-beta buildup and the progression of AD. These mice are a great way to study how ApoE helps clear amyloid-beta and prevent plaque formation. Without ApoE, the amyloid-beta piles up in the brain, accelerating neurodegeneration and cognitive decline. Researchers can also use these mice to explore how the different versions of ApoE, like ApoE4 affect disease progression, providing critical insights into why some people are at a higher risk of developing Alzheimer’s.
Using ApoE knockout mice, scientists can also examine other aspects of brain health, such as inflammation and synaptic damage. These studies help us understand the broader impact of ApoE deficiency on brain function and provide clues on how to stop the disease from progressing.
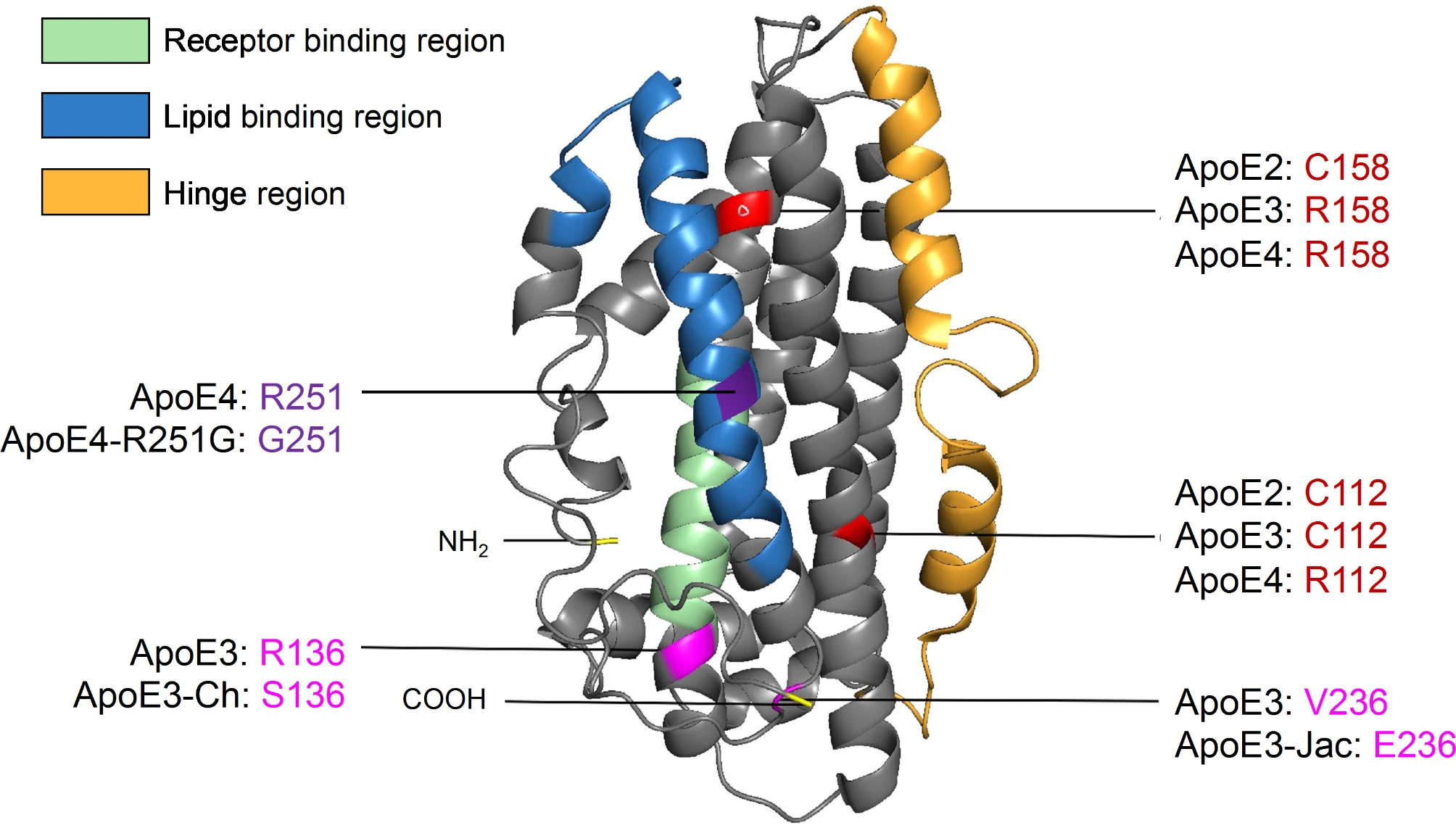
Structural model of apoE highlighting AD-related amino acid variations. ApoE is a 299 amino acid glycoprotein with a molecular weight of 34 kDa (PDB 2L7B). It is formed of two independently folded domains linked by a hinge region: the N-terminal domain (residues 1-167) contains the receptor-binding region while the C-terminal domain (residues 206–299) includes the lipid-binding region. There are three major apoE isoforms that differ at amino acid positions 112 and 158: apoE2 (C112/C158); apoE3 (C112/R158); and apoE4 (R112/R158). Additional rare apoE variants have been identified: apoE3-Christchurch (R136S), apoE3-Jacksonville (apoE3-V236E), and apoE4-R251G.
From: ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies
What Are the Benefits of Using ApoE Knockout Mice for Cardiovascular Research?
Beyond Alzheimer’s disease, ApoE knockout mice have made significant contributions to cardiovascular research. ApoE is essential for regulating cholesterol levels and ensuring that lipids are properly transported in the bloodstream. Without ApoE, these mice develop high cholesterol and other lipid imbalances, leading to the early development of atherosclerosis, a condition where plaque builds up in the arteries, increasing the risk of heart attacks, strokes, and other cardiovascular issues.
In these mice, atherosclerosis develops naturally, allowing scientists to study how lipid buildup, plaque formation, and inflammation contribute to heart disease. By observing how these processes unfold in the ApoE knockout mice, researchers gain critical insights into the causes of cardiovascular diseases, which are major killers worldwide.
ApoE knockout mice are also used to study conditions like heart attacks, stroke, and high blood pressure. These models help scientists understand how ApoE deficiency affects blood vessel function, smooth muscle cells, and artery flexibility—key factors in the development of heart disease. Thanks to these mice, researchers are identifying new ways to prevent or treat these conditions.
Moreover, ApoE knockout mice are frequently used to test potential treatments for cardiovascular diseases. For example, scientists can try out lipid-lowering drugs or anti-inflammatory treatments to see how they impact the progression of atherosclerosis in these mice. By testing new therapies in these models, researchers are helping pave the way for future treatments that could save lives.
Recent Research and Emerging Therapies
There is also exciting new research focused on modulating ApoE expression or even editing the ApoE gene to reduce the risk of Alzheimer’s and cardiovascular disease. By using techniques like gene therapy, researchers are exploring ways to either replace the missing ApoE or fix the problem caused by the ApoE4 variant. This could potentially be a game-changer in how we approach the treatment and prevention of these devastating diseases.
Conclusion
ApoE knockout mice are more than just a research tool—they are essential for advancing our understanding of both Alzheimer’s disease and cardiovascular health. These mice help scientists uncover the critical roles that ApoE plays in the body, from clearing amyloid-beta in the brain to regulating cholesterol and protecting the heart. By using these models, researchers can better understand how ApoE deficiencies lead to diseases, and how we might be able to intervene with targeted treatments. In the future, ApoE knockout mice could help us develop therapies that prevent or even reverse these debilitating conditions, improving the quality of life for millions of people around the world.
References
- Corder, E. H., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993 Aug 13;261(5123):921-3. doi: 1126/science.8346443.
- Holtzman, D. M., et al. (2000). Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2892-7. doi: 1073/pnas.050004797.
- Mahley, R. W. (2016). Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl). 2016 Jul;94(7):739-46. doi: 1007/s00109-016-1427-y.
- Plump, A. S.,et al. (1995). Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995:15:495-518. doi:1146/annurev.nu.15.070195.002431.
- Rebeck G. W., et al. (1993). Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993 Oct;11(4):575-80. doi: 1016/0896-6273(93)90070-8.
- Reddick, R. L., et al.(1994). Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994 Jan;14(1):141-7. doi: 1161/01.atv.14.1.141.

